Trying to make heads or tails of a lab report for a raw cannabinoid product? In this video, you will learn how to read your COA and make sure your bulk cannabinoid material meets your expectations.
A COA, or certificate of analysis, is a document cannabinoid producers use to provide technical information on their products. Specifically, a COA verifies that the product you are purchasing conforms to your requirements and meets your needs as a consumer. COAs are certified by an independent labs or are prepared by the producers themselves. Most certificates of analysis will contain specific details about the chemical analysis of a substance or in the case of cannabinoids, a profile.
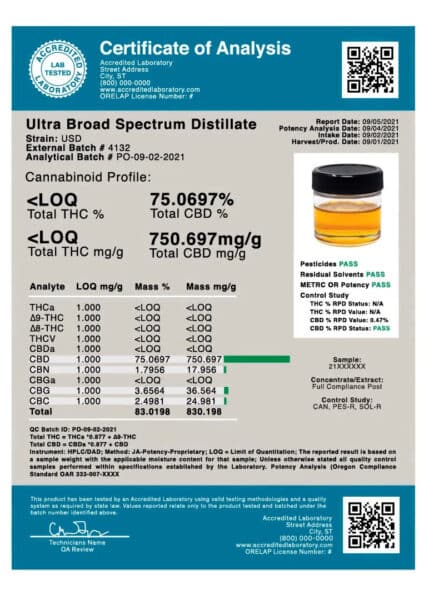
Bulk cannabinoid ingredient COAs provide information on, at the very least, the type of sample tested and the sample’s cannabinoid profile. Let’s cover some of the forms of information your raw material cannabinoid COA should include:
Testing date
Check the date on your COA first. You usually shouldn’t purchase cannabinoid raw materials that are more than 12-18 months old. Cannabinoids may degrade with time and the active compounds will lose potency and efficacy.
Batch number
The client batch number (or external batch number) is selected by the cannabinoid producer. You’ll need this important number to trace the batch associated with your test.
LOQ
In the testing industry, the limit of quantitation (LOQ) is the lowest threshold of a substance a test measures. Many CBD lab tests, for instance, set their THC LOQs at around 0.3% since that’s the federally designated THC cutoff for industrial hemp.
Total CBD % vs. total CBD mg/g
Each cannabinoid present in the sample will be listed both as a percentage and as a total milligram sum. You can use this information to make sure your raw material contains the cannabinoid concentrations advertised.
For example, if a 1g sample of distilled CBD concentrate advertised as containing 75% CBD has a lab report pegging its CBD concentration at around 750mg per gram, then the advertised concentration is correct. However, if a CBD lab report advertises that a concentrate contains 75% CBD with a CBD concentration at 75 mg per gram, than the advertised concentration is incorrect.
Cannabinoid breakdown
Make sure to check the concentrations of each minor cannabinoid. With the popularity of minor cannabinoids like CBG, CBN, and CBC on the rise, you can use this potency information to determine how strongly your finished product will express the attributes of each cannabinoid.
Calculating product formulations
Use the potency information listed on your report to start planning bulk cannabinoid product formulations. Simply determine how much CBD your product contains per kilo, and divide the total number of milligrams of CBD you wish to purchase by the number of CBD milligrams you want each finished product to contain to find out how many total products you can make.
For instance, if you purchase one kilo of CBD Isolate and want to use it to formulate 500mg tinctures, you will be able to make roughly 2,000, 500mg tinctures from the single kilo of isolate.

Additional acronyms you may need to know:
PPM: stands for parts per million
PPB: stands for parts per billion
LOD: stands for “limit of detection” and is similar to the LOQ, but they are not exactly equivalent. The LOD is the lowest quantity of a substance that can be distinguished from the absence of that substance. The LOD and LOQ can differ according to what definition is used and what type of noise contributes to the measurement and calibration.
<LOQ: means that the analyte is below or less than the limit of quantitation.
California: may be specified on certain COAs. In California, the limit of quantitation may be above the limit of detection, meaning the analyte is detectable, but not necessarily higher than the LOQ.
ND: means not detected
NT: stands for not tested
Mg/g: is milligrams per gram. If you divide this number by 10 you get the percent concentration

Limit/action limit: This is the limit set by state regulators and can differ from state to state. If the COA shows a result above the Limit or Action Limit it represents a failing result.
We hope we’ve answered all your questions and concerns about raw material COAs. If you have any further inquiries about COAs, sourcing high-quality cannabinoids, or our white label services please book a call with one of our industry experts.
COA FAQs
1. What is in a COA?
COAs for hemp and cannabis products generally consist of a few important pieces of information regarding:
- The cannabinoid and terpene concentrations in the sample
- Concentrations of any contaminants that may be present
- A unique number used to identify the sample
- Photographs of additional identifying material
As we’ve covered in the guide above, a COA also includes a lot of other data that you’ll need to parse over to get a full idea of what’s in your product. Knowing the cannabinoid concentrations and how to identify the sample, though, is usually everything the average shopper is after.
2. How do you read a COA for CBD?
COAs for CBD and other cannabinoid products are generally designed to be easy to read with the most relevant information provided the most clearly in the largest print. An average COA for CBD distillate, for instance, will feature the CBD concentrations front and center — in both milligrams and percentile. If you have any questions about how to read a COA, contact the laboratory that issued it.
3. How can you tell if CBD is high-quality?
Referencing a COA is the easiest and most reliable way to establish the potency and safety of a CBD product — in other words, its quality. Simply making COAs easy to find is a step in the right direction, and the lab report itself should check all the boxes.
4. How do you know if a COA is real?
The easiest way to establish the authenticity of a COA is to contact the issuing lab. If there is no lab listed on the COA, that’s an instant red flag.








