Autism spectrum disorder (ASD) is a complex neurodevelopmental condition marked by challenges in social interaction, communication, repetitive behaviors, and restricted interests. Current treatments range from behavioral therapies to pharmaceuticals, but many children diagnosed with severe ASD remain resistant to these conventional options. Families and healthcare providers are continuously searching for safer, effective alternatives to enhance quality of life and symptom management.
A study recently published in the respected journal Pharmacology Biochemistry and Behavior sheds new light on the potential of purified cannabidiol (CBD) as an effective therapeutic strategy for children suffering from severe, treatment-resistant ASD. The study provides rigorous evidence of CBD’s ability to improve core symptoms and reduce problematic behaviors, offering considerable hope for those not benefiting from standard therapies.
Understanding the Scope of the Challenge
Autism spectrum disorder encompasses a wide range of presentations, from mild social difficulties to profound impairments affecting all aspects of daily life. For individuals at the severe end of this spectrum, daily challenges often include heightened irritability, aggressive outbursts, severe hyperactivity, repetitive behaviors, and communication difficulties. Unfortunately, many of these children respond inadequately to traditional therapies and medications, leaving families and caregivers with limited therapeutic options.
Existing pharmaceutical interventions often come with adverse effects that can sometimes outweigh their benefits. Consequently, there is significant demand within the medical community for alternative treatments that combine safety with effectiveness, particularly for the pediatric population.
Exploring the Therapeutic Potential of Cannabidiol (CBD)
CBD, a non-intoxicating cannabinoid derived from the cannabis plant, has been studied extensively over recent years for its potential therapeutic applications. CBD doesn’t cause intoxication like THC, making it appealing for pediatric use. Prior studies have suggested CBD’s efficacy in managing epilepsy, anxiety, chronic pain, and various neurological disorders, which prompted researchers to explore its potential impact on autism spectrum disorder.
The new research from Pharmacology Biochemistry and Behavior aimed specifically to evaluate the effectiveness and tolerability of purified CBD for children diagnosed with severe ASD whose symptoms were resistant to conventional treatments.
The Study: Assessing CBD’s Impact on Severe Autism Spectrum Disorder
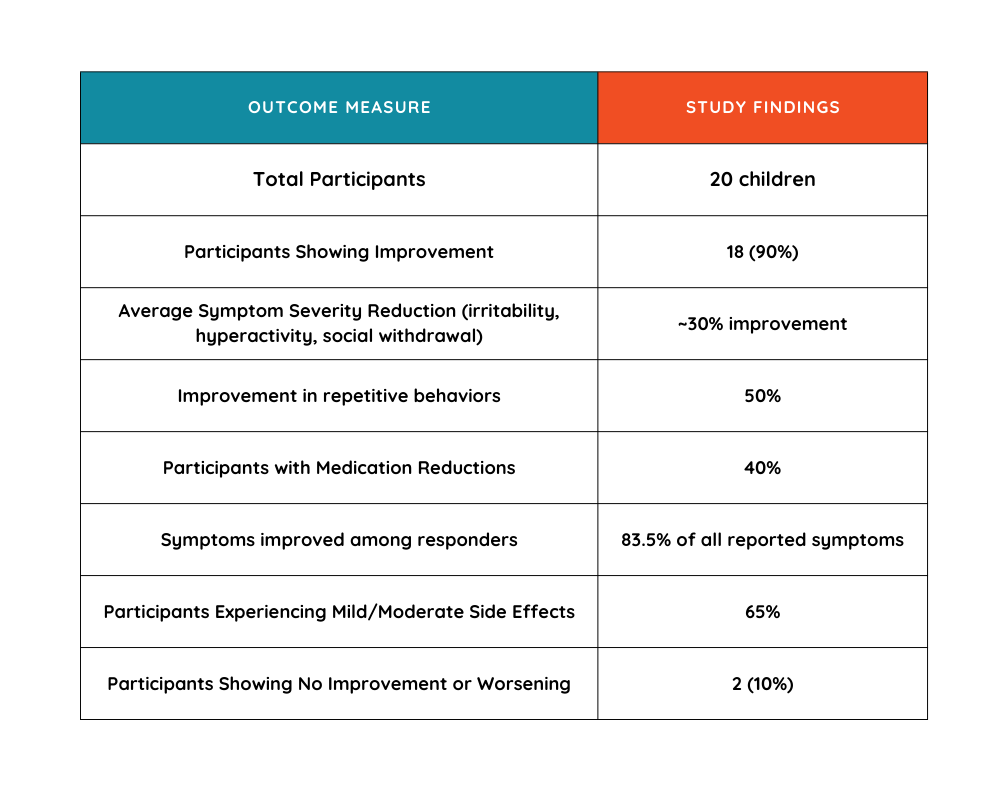
The study involved 20 pediatric patients diagnosed with severe ASD, all of whom had not responded adequately to conventional treatments. Over a period of three months, participants received purified CBD, with assessments conducted at baseline and at three-month intervals to evaluate changes in behavior, symptom severity, and overall well-being. The range of CBD dose was 100-700mg, with a median total CBD dose of 363.5mg.
Results demonstrated that treatment with purified CBD led to notable improvements in several core symptoms of ASD. These included reductions in irritability, aggression, and hyperactivity, as well as enhancements in communication and social interaction. Importantly, the treatment was well-tolerated, with minimal adverse effects reported.
Key Study Findings
- Parental Reports of Symptom Improvement: Parents reported symptom improvements in 90% (18 out of 20) of participants following CBD treatment initiation.
- Among these responders, an impressive 83.5% of all reported symptoms showed improvement, highlighting significant and broad-spectrum symptom relief. Only two participants (10%) saw no benefit, with one experiencing no response and one experiencing symptom worsening.
- Average Severity Reduction: Among participants who experienced improvement, core ASD symptoms—including irritability, social withdrawal, and hyperactivity—decreased in severity by an average of 30% from their baseline measurements. This indicates a meaningful reduction in symptom intensity across the group.
- Reduction of Repetitive Behaviors: A substantial 50% of participants exhibited measurable improvement in repetitive and restricted behaviors, which are notoriously resistant to conventional therapeutic strategies. Only about 39% showed no change, indicating CBD’s potent ability to reduce these particularly challenging symptoms.
- Reduction in Medication Dependence: Remarkably, 40% of children taking part in the study experienced a reduction or partial discontinuation of other concurrent medications. CBD may decrease dependence on traditional ASD medications, lowering overall drug exposure and side effects.
- Safety and Tolerability: Safety is a significant concern when introducing new treatments for pediatric populations. Encouragingly, CBD was well-tolerated among the study’s participants, with mild-to-moderate side effects occurring in 65% of cases, predominantly transient irritability or decreased appetite. These side effects resolved without intervention, and no participants discontinued CBD due to adverse effects.
Implications for Future Treatment Strategies
The findings from this research provide compelling evidence supporting CBD’s integration into treatment strategies for severe ASD, particularly when traditional interventions prove inadequate. The substantial improvement in key symptom domains, coupled with CBD’s excellent safety profile, underscores its potential role as an adjunctive or alternative therapeutic strategy.
Nonetheless, it’s crucial to approach these promising results with informed optimism. Larger, longer-term randomized controlled trials are needed to confirm these findings. These future studies should aim to establish optimal dosing, elucidate the precise mechanisms of CBD’s neurological effects, and assess long-term safety and efficacy.
The potential reduction in medication reliance observed in this study also opens exciting avenues for further research into CBD’s role in pharmacological management strategies, potentially leading to more streamlined, safer, and more effective treatment plans.
Conclusion: A Promising Step Forward for Autism Treatment
This landmark study significantly expands our understanding of CBD’s therapeutic possibilities in managing severe autism spectrum disorder. More research is needed, but this study clearly shows CBD can greatly improve life quality in treatment-resistant ASD.
At GVB Biopharma, we recognize the critical importance of rigorous scientific exploration into cannabinoids like CBD, committed to providing high-quality, pure CBD extracts and formulations. As research advances, the prospects of integrating CBD into standardized therapeutic practices become increasingly promising, underscoring the ongoing need for high-quality, scientifically validated cannabinoid products.
Families, healthcare providers, and the broader ASD community can view these results as an encouraging indication that effective alternative treatments are within reach. With further investigation and careful application, CBD could soon become a cornerstone in the management of severe ASD, bringing new hope and relief to countless families facing this challenging condition.